Anode-free lithium metal batteries, which have attracted attention as candidates for electric vehicles, drones, and next-generation high-performance batteries, offer much higher energy density than conventional lithium-ion batteries. However, their short lifespan has made commercialization difficult.
KAIST researchers have now moved beyond conventional approaches that required repeatedly changing electrolytes and have succeeded in dramatically extending battery life through electrode surface design alone.
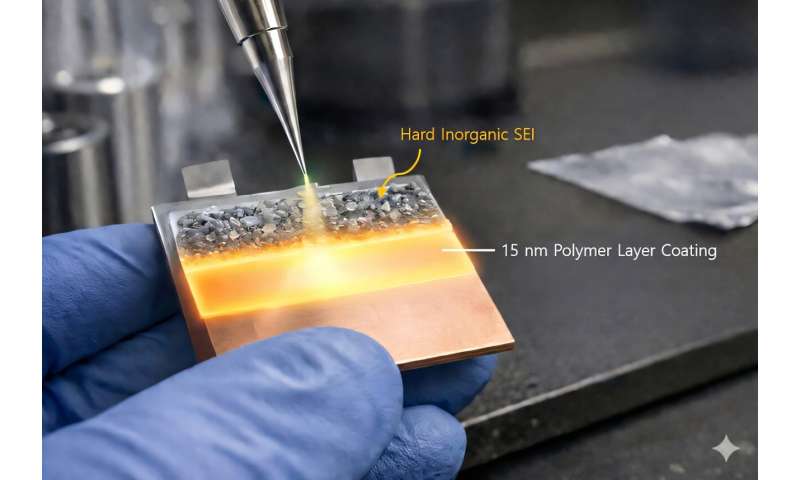
A research team led by Professors Jinwoo Lee and Sung Gap Im of the Department of Chemical and Biomolecular Engineering fundamentally resolved the issue of interfacial instability—the greatest weakness of anode-free lithium metal batteries—by introducing an ultrathin artificial polymer layer with a thickness of 15 nanometers (nm) on the electrode surface.
The results are published in Joule.
How anode-free batteries work
Anode-free lithium metal batteries have a simple structure that uses only a copper current collector instead of graphite or lithium metal at the anode. This design offers advantages such as 30–50% higher energy density compared to conventional lithium-ion batteries, lower manufacturing costs, and simplified processes.

However, during the initial charging process, lithium deposits directly onto the copper surface, rapidly consuming the electrolyte and forming an unstable solid electrolyte interphase (SEI), which leads to a sharp reduction in battery lifespan.
Rather than changing the electrolyte composition, the research team chose a strategy of redesigning the electrode surface where the problem originates.
By forming a uniform ultrathin polymer layer on the copper current collector using an iCVD (initiated chemical vapor deposition) process, they found that this layer regulates interactions with the electrolyte, precisely controlling lithium-ion transport and electrolyte decomposition pathways.
In conventional batteries, electrolyte solvents decompose to form soft and unstable organic SEI layers, causing non-uniform lithium deposition and promoting the growth of sharp, needle-like dendrites.
In contrast, the polymer layer developed in this study does not readily mix with the electrolyte solvent, inducing the decomposition of salt components rather than solvents.
As a result, a rigid and stable inorganic SEI is formed, simultaneously suppressing electrolyte consumption and excessive SEI growth.
Mechanism and industrial potential
Using operando Raman spectroscopy and molecular dynamics (MD) simulations, the researchers identified the mechanism by which an anion-rich environment forms at the electrode surface during battery operation, leading to the formation of a stable inorganic SEI.
This technology requires only the addition of a thin surface layer without altering electrolyte composition, offering high compatibility with existing manufacturing processes and minimal cost burden. In particular, the iCVD process enables large-area, continuous roll-to-roll production, making it suitable for industrial-scale mass production beyond the laboratory.
Professor Jinwoo Lee stated, "Beyond developing new materials, this study is significant in that it presents a design principle showing how electrolyte reactions and interfacial stability can be controlled through electrode surface engineering. This technology can accelerate the commercialization of anode-free lithium metal batteries in next-generation high-energy battery markets such as electric vehicles and energy storage systems (ESS)."
This research was conducted with Ph.D. candidate Juhyun Lee, and postdoctoral Jinuk Kim, a postdoctoral researcher from the Department of Chemical and Biomolecular Engineering at KAIST, serving as co–first authors.
More information: Juhyun Lee et al, A strategic tuning of interfacial Li+ solvation with ultrathin polymer layers for anode-free lithium metal batteries, Joule (2025). DOI: 10.1016/j.joule.2025.102226
Citation: Ultrathin polymer layer extends lifespan of anode-free lithium metal batteries (2026, January 5) retrieved 5 January 2026 from https://techxplore.com/news/2026-01-ultrathin-polymer-layer-lifespan-anode.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.