Hydrogen, the lightest element on the periodic table, is a master of escaping almost any container it's stored in. Its extremely small size allows it to squeeze through atomic-scale gaps in the storage materials, which is one of the major issues hindering hydrogen energy from becoming mainstream.
A team of Chinese researchers has solved the issue of containment with on-demand hydrogen production. They developed a simple chemical system containing commercial ammonium metatungstate (W12) and graphitic carbon nitride (g-C3N4) in a liquid suspension. This system captures solar energy and, rather than converting it into electricity, uses it to produce hydrogen fuel on demand—even in darkness.
The new system provided twofold benefits: it made solar energy available even when the sun isn't shining, and it eliminated the need to transport hydrogen in dangerous, high-pressure tanks.
Per the findings published in Advanced Materials, this technology achieved impressive hydrogen production rates, generating 3,220 µmol g−1 h−1 in darkness and 954 µmol g−1 h−1 under natural outdoor sunlight, both impressive figures when compared to the output of similar dark photocatalytic systems.

Trapping the photons
Plants have mastered a remarkable balancing act in photosynthesis. They separate light-driven and dark reactions, capturing energy from sunlight first, then using that stored energy later to power chemical processes without the sun.
Inspired by this strategy, scientists have been working to build artificial photosynthesis systems that mimic this approach, in hopes of creating a greener way to produce hydrogen using solar power that continues working even after the sun goes down.
Polyoxometalates, or POMs, are a class of metal–oxygen clusters known for their ability to store and release multiple electrons, making them a promising candidate for this approach. So far, only a handful of POM-based photocatalytic systems have produced hydrogen without light, and most rely on complex, difficult-to-build catalytic setups that often require an external electrical input.

Hydrogen production goes dark-mode
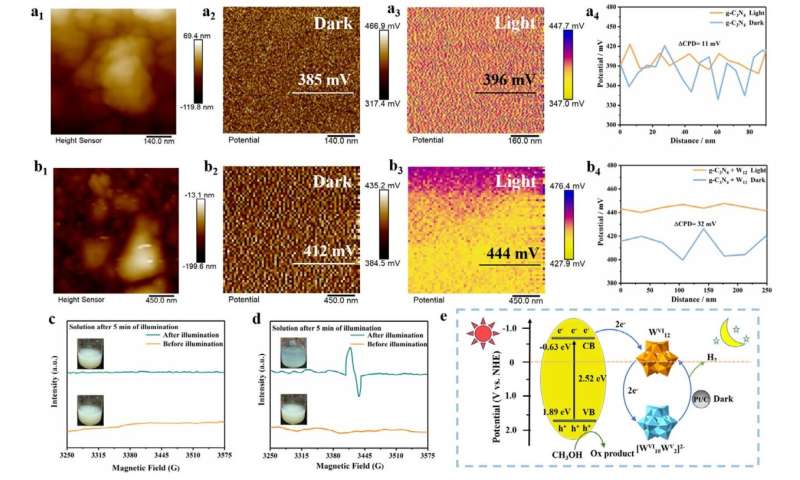
The researchers in this study addressed this issue by integrating a graphitic carbon nitride semiconductor with commercial POM, ammonium metatungstate, in a methanol solution via electrostatic assembly. This gave rise to a suspension that could support both the light phase for energy capture and the dark phase for driving chemical reactions and releasing hydrogen internally.
When exposed to sunlight, the liquid system absorbed light through graphitic carbon nitride, the light harvester. This process triggered electron production and the electrons formed found their way to tungsten POM, which acted as an electron storage medium. A visible shift in the color of the solution from pale yellow to blue signals that energy has been stored.
The captured energy remained safely stored until fuel was needed. When on-demand hydrogen production was required, the team introduced a platinum-based catalyst, which released the stored electrons and triggered the chemical reactions that produced hydrogen gas.
The team achieved excellent hydrogen production rates under both dark conditions and natural sunlight. Further analysis into the mechanism showed that the two materials chosen had perfect energy matching, and when they came into contact, they created an internal electric field, which allowed electron transfer at record speeds, which also amplified the dark phase hydrogen production.
The researchers believe this new system has demonstrated its ability to reliably produce hydrogen under all weather conditions and shows strong potential for practical scaling. With further development, this approach could allow hydrogen to be safely produced in sun-rich regions and delivered to energy-poor areas, without relying on dangerous high-pressure storage.
Written for you by our author Sanjukta Mondal, edited by Lisa Lock, and fact-checked and reviewed by Robert Egan—this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a donation (especially monthly). You'll get an ad-free account as a thank-you.
More information: Xiaoyu Dong et al, Solar Energy Storage in Polyoxometalate for On‐Demand Hydrogen Transportation and Evolution, Advanced Materials (2025). DOI: 10.1002/adma.202519875
© 2026 Science X Network
Citation: On-demand hydrogen fuel production goes dark-mode (2026, January 2) retrieved 3 January 2026 from https://techxplore.com/news/2026-01-demand-hydrogen-fuel-production-dark.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.